-

Socicalal Singete jz-zms3
-

Socicalulal Singeter Jz-zms4
-

Socicalal Sidbive Jz-zms5
-

Socicalal Sidbive Jz-zms9
-

Hoos udhaca joliga ah jz-zt
-

Socicalal Singete jz-az
- Sifo
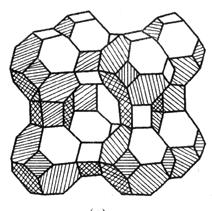
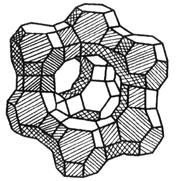
| Qaaciddada kiimikada ee unugyada zeolite | Mx / n [(alo.2) x (Sio.2) y] wh.2O. |
| MX / n. | Cation ion, adoo ku hayaya crystal elektrally dhexdhexaad |
| (Alo2) X (Sio2) y | Lafaha ka mid ah kiristaaga zeolite, oo leh qaabab kala duwan oo godad iyo kanaalo ah |
| Haa | Jir ahaan uumiga jirka |
| Astaamaha | ADSOSORORASTIOROPASTIONTIIN WAA IN LAGU SAMEEYAA |
| Nooca shaandhaynta amolecaular | | |
| Nooca Xabfaha Xariirka |
| Qeybta ugu weyn ee Xabsiga Molicon-ka waa Silicon Calaamad, godka ugu weyn ee Crystal waa laba iyo tobanka qaddarka ah oo ah scystel-ka '' scroulal styral ' Aperture oo ah 8-9 a, oo loo yaqaan 10x (oo sidoo kale loo yaqaan kaalshiyamka x) godka muruqyada. |
- Isticmaal
- Qaybinta apertular ee murqaha 'midecular' waa labis aad u lebis, iyo walxaha oo keliya dhexroor molital ah oo ka yar dhexroorkii godka ayaa geli kara godka 'crystal' ee gudaha miraha.